NAFDAC has raised alarm over killer cough syrups from India.
Glamtush reports that the National Agency for Food and Drug Administration and Control (NAFDAC) on Tuesday raised alarm over the use of four cough syrups linked to acute kidney injuries and children’s deaths in The Gambia.
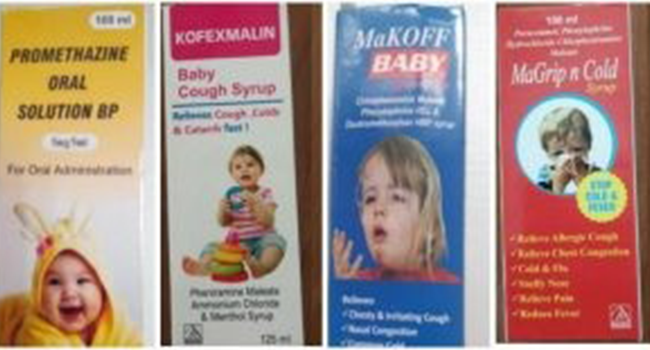
According to the World Health Organisation, the four products are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
The products are manufactured by an Indian company, Maiden Pharmaceuticals, which had failed to provide guarantees about their safety, NAFDAC Director-General, Prof. Mojisola Adeyeye, said at a briefing.
NAFDAC said although the drugs are not expected to be in Nigeria – as it has not been ratified by the agency – Nigerians should stay vigilant as it “may have been distributed, through informal markets, to other countries or regions.”
See the full text briefing of the NAFDAC DG below:
Press Briefing On The Substandard (Contaminated) Paediatric Cough Syrups Circulating In Gambia
October 11, 2022,
Protocol
Gentlemen and ladies of the Press
On 5th of October 2022, the World Health Organization (WHO) issued a global alert for over four (4) cough syrups – warning they could be linked to acute kidney injuries and the children’s deaths in July, August, and September in The Gambia.
Interviews conducted on bereaved parents in The Gambia by health authorities and law enforcement agencies revealed how their children are not able to pass urine after being given the syrups. As their condition worsened, efforts to save their lives were fruitless which has resulted in the death of over 66 children. This is a worrisome development that once again beams a searchlight on the essence of effective regulation and control of medical products.
As a member of the WHO Programme on International Drug Monitoring and following our active participation in the WHO member state mechanism on substandard and falsified medical products, NAFDAC is leaving no stone unturned in ensuring that these products do not cause harm to our people. As part of our regulatory controls, NAFDAC have issued an alert to the public on the Public Alert No. 039/2022 – Alert on Substandard (contaminated) paediatric cough syrups circulating in Gambia. This alert has been widely circulated on our website and all social media handles and sent to all health care providers on our database as well as professional bodies and association in the medicine supply chain.
In addition, we have put appropriate measures in place to prevent entry of these spurious products from the various ports of entry and have activated our internal surveillance mechanisms to mop up these products from the supply chain pipeline if they are ever found.
These suspected medical products – Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup – were manufactured by an Indian company, Maiden Pharmaceuticals, which had failed to provide guarantees about their safety.
The National Agency for Food and Drugs Administration and Control (NAFDAC) is using these medium to further notify healthcare providers and awaken the consciousness of the public on these four substandard products, identified in The Gambia and reported to WHO in September 2022.
The four products are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
According to WHO report, laboratory analysis of samples of the four products confirms that they contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.
Diethylene glycol and ethylene glycol are restricted chemicals which are toxic to humans when consumed and can prove fatal. These chemicals are closely related in chemical structure to propylene glycol which is the right vehicle for use in the manufacture of paediatric syrups. Poor quality control might have led to non-detection of these dangerous by products and hence the fatality we have on our hands in the Gambia.
The notable examples of drug quality problems resulting to serious public consequences and highlighting the significance of NAFDAC include: the disaster of poorly compounded Chloroquine syrup at UNTH Enugu in 1990, Paracetamol syrup disaster at UCH Ibadan and JUTH in 1990, fake meningitis vaccine circulating in Northern Nigeria in 1996, reported cases of contaminated infusions in circulation in 2002, and the “My Pikin” saga in 2008 that was a result of negligence in manufacturing, resulting in poorly formulated Paracetamol syrup. Under my watch as Director-General (NAFDAC), we are working assiduously to ensure this does not happen again in our dear country.
It is pertinent to note that toxic effects of this contamination in cough syrup include abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.
The stated manufacturer of these products is Maiden Pharmaceuticals Limited (Haryana, India). To date, the stated manufacturer has not provided guarantees to WHO on the safety and quality of these products.
According to WHO, the four cold and cough syrups in question have been potentially linked with acute kidney injuries and 66 deaths among children in Gambia.
Product Details
The details of the substandard cough syrups are as follows;
| Product Manufacture | Product Name |
| Maiden Pharmaceuticals Limited (Haryana, India) | · Promethazine Oral Solution,· Kofexmalin Baby Cough Syrup,
· Makoff Baby Cough Syrup · Magrip N Cold Syrup |
All batches of these products listed above should be considered unsafe. The substandard products in this alert are unsafe and their use, in children and adults will result in serious injury or death.
These products are not registered by NAFDAC, therefore should not be in circulation.
To date, these four products have been identified in The Gambia, but may have been distributed, through informal markets, to other countries or regions. That is why we have heightened our vigilance.
NAFDAC implores importers, distributors, retailers and consumers and stakeholders to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the substandard cough syrups. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Members of the public in possession of the above listed products are advised to discontinue sale or use and submit stock to the nearest NAFDAC office.
If you have these substandard products, please DO NOT use them. If you, or someone you know, have used these products, or suffered any adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional.
Healthcare professionals, consumers and members of the public are encouraged to report any suspicion of adverse drug reaction and substandard and falsified medicines to NAFDAC on 0800-162-3322 or email: sf.alert@nafdac.gov.ng
NAFDAC………safeguarding the health of the Nation!!!
Director General